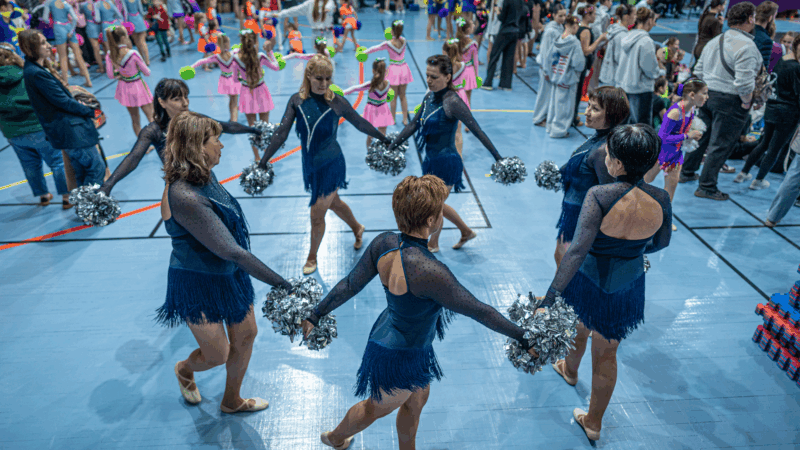Vaccine advisers to the FDA recommended changes to COVID vaccines
The companies that make COVID-19 vaccines should update the shots again to target a variant closer to the strains currently on the rise, a committee of independent advisers to the Food and Drug Administration unanimously recommended Thursday.
Moderna, Pfizer/BioNTech and Novavax should target strains related to the JN.1 variant with their vaccines for next fall and winter because that strain is closer to the new variants of the virus that are circulating, the advisers voted after a day-long meeting.
The recommendation came after the companies presented data that shows that vaccines based on viruses related to JN.1 strains produce strong protection against the latest versions of the virus, such as LP.8.1, which has become dominant in the U.S.
The current Moderna and Pfizer/BioNTech’s mRNA vaccines target the KP.2 strain. Novavax’s shot targets a JN.1 strain.
The committee was uncertain about which particular JN.1 subvariant would be the best pick because it isn’t known which strain may be dominant by the fall. The FDA will now work with the companies to make a final decision, officials said.
The advisory panel’s vote is consistent with the World Health Organization’s recommendation for the next set of vaccines.
The decision underscores the state of the evolution of the virus, which has now essentially become endemic in the U.S. and other countries. The virus continues to produce new subvariants of omicron instead of evolving into dramatically different strains that would pose a greater risk.
Changes in the FDA approval process
The recommendation comes two days after the FDA announced a new approach to COVID vaccines that would likely restrict access to the shots. While many questions remain about the new strategy, a change in strains would not appear to make a difference in how the FDA approves the next vaccines.
The new strategy would continue the current vaccine approval process for people ages 65 and older and younger people with health problems that put them at high risk for serious complications from the virus. Those health problems include obesity, heart disease, cancer, inactivity and other risk factors. That regulatory approach relies on information about how the immune system responds to the vaccines.
But the FDA will now require vaccine manufacturers to conduct large, costly additional studies to evaluate the safety and effectiveness of the vaccines for children and younger healthy adults by comparing them to a harmless injection of saline. Those trials would look at differences in health outcomes, such as developing COVID.
That’s a major change from the current approach, which recommends and approves the vaccines for almost everyone based on the more easily obtained immune system studies.
FDA officials say the change was prompted by the widespread immunity to the virus that people have developed because of repeated infections and vaccinations. This acquired immunity has contributed to a significant drop in serious illness and death from COVID. The FDA estimates 100 million to 200 million Americans would be eligible for COVID vaccines under the new approach.
Some independent experts are welcoming the change. Others, however, worry the move would make it harder for many people who still want the vaccine to get it. That would include parents who want to vaccinate very young children and those who want to reduce their risk for mild or moderate illness, long COVID and the risk of spreading the virus to other people, such as older relatives.
FDA officials say the steps will bring the U.S. in line with the approach that other high-income countries take towards the vaccines and are necessary to restore trust in the vaccines.
Even though COVID is still claiming more lives than the flu, most U.S. adults have declined to get vaccinated against COVID in recent years and even fewer parents have opted to vaccinate their children. Children tend to be far less likely to get seriously ill from COVID, but the disease can still be serious for them, especially very young children.
Bill making the Public Service Commission an appointed board is dead for the session
Usually when discussing legislative action, the focus is on what's moving forward. But plenty of bills in a legislature stall or even die. Leaders in the Alabama legislature say a bill involving the Public Service Commission is dead for the session. We get details on that from Todd Stacy, host of Capitol Journal on Alabama Public Television.
Baz Luhrmann will make you fall in love with Elvis Presley
The new movie is made up of footage originally shot in the early 1970s, which Luhrmann found in storage in a Kansas salt mine.
My doctor keeps focusing on my weight. What other health metrics matter more?
Our Real Talk with a Doc columnist explains how to push back if your doctor's obsessed with weight loss. And what other health metrics matter more instead.
Forget the State of the Union. What’s the state of your quiz score?
What's the state of your union, quiz-wise? Find out!
A team of midlife cheerleaders in Ukraine refuses to let war defeat them
Ukrainian women in their 50s and 60s say they've embraced cheerleading as a way to cope with the extreme stress and anxiety of four years of Russia's full-scale invasion.
SNL mocked her as a ‘scary mom.’ In the Senate, Katie Britt is an emerging dealmaker
Sen. Katie Britt, Republican of Alabama, is a budding bipartisan dealmaker. Her latest assignment: helping negotiate changes to immigration enforcement tactics.