Scientists have re-created a pain pathway in the brain by growing four key clusters of human nerve cells in a dish.
This laboratory model could be used to help explain certain pain syndromes, and offer a new way to test potential analgesic drugs, a Stanford team reports in the journal Nature.
“It’s exciting,” says Dr. Stephen Waxman, a professor at Yale School of Medicine who was not involved in the research.
Currently, prospective pain drugs are typically tested in animals — whose responses are often different than a human’s — and in individual nerve cells, which may not reflect the behavior of entire brain networks.
With this new system, known as a brain assembloid, “we have a miniature nervous system that might be a very useful platform,” Waxman says.
A pathway with several stops
The model is the result of an effort to re-create the signaling chain that occurs after exposure to painful stimuli, says Dr. Sergiu Pașca, a professor at Stanford University who led the project.
Touch a hot stove, for example, and special cells in the skin “send that information all the way to the spinal cord,” Pașca says. “Then the spinal cord will relay it up to the thalamus deep in the brain, and then all the way to the outer layer of the brain, which is the cortex.”
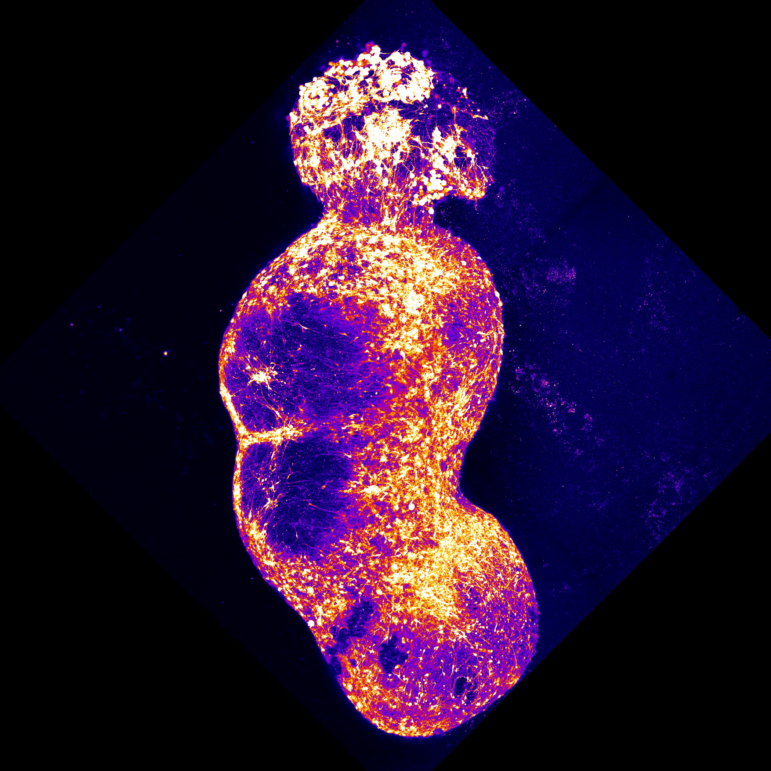
To approximate this pathway in the lab, Pașca’s team created four different brain organoids, spherical clumps of human nerve cells that grow in a dish. The team coaxed each organoid to resemble one specific type of brain or spinal tissue found along the pain pathway.
“And then we put them together, really put them in close proximity, and watched them as they connected with each other,” Pașca says.
After more than six months developing in the lab, the resulting assembloid had created a pathway linking the four organoids. The nerve cells also spontaneously began “working in a coordinated fashion across the four parts of this assembloid,” Pașca says.
Chili peppers and pain syndromes
To test the model, the team exposed it to capsaicin, the chemical that makes chili peppers painfully hot.
“Then you start seeing that information traveling,” Pașca says. “The neurons that sense these signals get activated and they transmit that information to the next station and the next station, all the way to the cortex.”
Next, the scientists tried creating assembloids using cells with genetic variants linked to abnormal pain perception.
One of these variants causes a rare condition called erythromelalgia, or man-on-fire syndrome.
“These individuals feel searing, burning, scalding pain in response to mild warmth,” Waxman says.
The scientists found that assembloids with the gene variant produced much more spontaneous communication between organoids, suggesting a heightened sensitivity to pain.
Results like that suggest that organoids are already a useful way to study both nervous system diseases and the pathways they affect, says Dr. Guo-li Ming, a professor at the University of Pennsylvania’s Perelman School of Medicine who also had no role in the new study.
For all its complexity, the pain pathway in a dish is a highly simplified version of what goes on in a person, Ming adds. For example, humans have two major pathways that carry pain signals to the brain, while the model system includes just one.
As a result, the model can detect a painful stimulus, but doesn’t produce an emotional response, Pasca says.
“So we don’t believe that this pathway that we’ve built is in any way feeling pain,” he says.
And these clusters of human cells are likely to become even more valuable as scientists find ways to re-create larger and more complex parts of the nervous system.
For example, Ming’s own lab has developed a model of a human neural tube, the structure in an embryo that eventually becomes into a baby’s brain and spinal cord. Her goal is to understand how neurological disorders develop early in life.
Transcript:
AILSA CHANG, HOST:
So when you touch a hot stove, the nerve endings in your fingers react instantly. But the ouch comes a split-second later, when that information finally reaches your brain. Well, now, scientists have reconstructed one of the sensory pathways that convey these pain signals all in a dish. NPR’s Jon Hamilton reports.
JON HAMILTON, BYLINE: Pain signals often start on the body’s surface. Then, says Dr. Sergiu Pasca of Stanford University, they make a long journey.
SERGIU PASCA: Nerve terminals in the skin send that information all the way to the spinal cord. And then the spinal cord will relay it up to the thalamus and then all the way to the outer layer of the brain, which is the cortex.
HAMILTON: Where the signals register as pain – Pasca wanted to recreate this pathway in the lab. So his team created four different brain organoids, spherical clumps of human nerve cells that grow in a dish. Pasca says the team coaxed each organoid to resemble one specific type of brain or spinal tissue.
PASCA: And then we put them together, really put them in close proximity, and watched them as they connected with each other.
HAMILTON: Much the way nerves in the skin connect with the spinal cord, which connects with the brain – the result, which took more than six months to build, created a pathway linking four organoids. Pasca calls it an assembloid. He says it began communicating spontaneously.
PASCA: The cells are just working in a coordinated fashion across the four parts of this assembloid.
HAMILTON: To test their creation, Pasca’s team expose the nerve endings on one organoid to the chemical that makes chili peppers painfully hot.
PASCA: Then you start seeing that information traveling. The neurons that sense the signals get activated, and they transmit that information to the next station and the next station, all the way to the cortex.
HAMILTON: Pasca says the model is designed to detect a painful stimulus but doesn’t link to the brain areas that cause an emotional response to discomfort.
PASCA: So we don’t believe that this pathway that we’ve built is in any way, like, feeling pain.
HAMILTON: The pathway in a dish is described in the journal Nature. Pasca says it offers a way to study how pain signals travel through the body and perhaps how to block them. Dr. Stephen Waxman of Yale University, who was not connected to the research, says the model could give researchers a new way to test potential pain drugs.
STEPHEN WAXMAN: We generally study them in single cells and then in whole animals. But here we have a miniature nervous system that might be a very useful platform.
HAMILTON: Waxman says the model also might help researchers understand a rare genetic condition he studies. It’s called man on fire syndrome.
WAXMAN: These individuals feel searing, burning, scalding pain in response to mild warmth – putting on a sweater, wearing shoes, mild exercise or going outside when it’s 72 degrees Fahrenheit.
HAMILTON: The condition is caused by a gene mutation. When Pasca’s team tried including this mutation in their pain pathway, it became much more sensitive to stimuli. Dr. Guo-li Ming of the University of Pennsylvania says the pain pathway model is useful but has limitations. For example, in a dish, signals travel only a fraction of an inch. Ming says that’s very different than what happens in a human body.
GUO-LI MING: It can be a meter long – right? – from your foot to your spinal cord. So definitely there’s a lack of structural organization.
HAMILTON: Even so, Ming says organoids are giving scientists a new way to study parts of the nervous system. Her own lab, for example, has created a model of a human neural tube, the structure in an embryo that becomes the brain and spinal cord. Jon Hamilton, NPR News.
(SOUNDBITE OF RHIAN SHEEHAN’S “LA BOITE A MUSIQUE”)